If asked to pinpoint the experiences that cemented my interest in immunology and microbiology, I would have to point to a semester spent studying abroad in Hong Kong. Despite being a zoologist, I was able to pick up a biomedical major and study a range of emerging and infectious diseases, including MERS and Chikungunya which I was barely aware of at the time. Viral diseases featured most heavily, as in such a densely populated city the lessons learnt from both the Asian and Hong Kong flu pandemics alongside the SARs outbreak have not been forgotten.
 |
One of several posters on the Man Cheung Po trail,
|
During this time, it was Dengue virus (DENV) that really caught my attention as an emerging disease. It wasn’t uncommon when hiking in the outer islands to come across signs warning of recent cases in the area, so when a new paper by Screaton et al emerged this September in Nature Immunology featuring both Dengue and zika I was intrigued. Whilst these flaviviruses are in no means new, a recent emergence in geographical range and viral pathogenesis have boosted these once obscure viruses to the forefront of biomedical research.
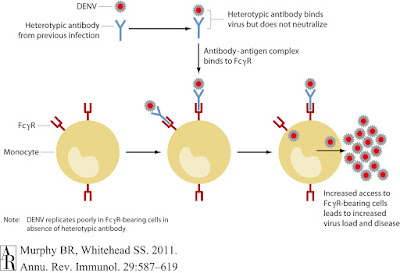
The birth defects and microcephaly accompanying the 2015 - 2016 Zika outbreak in Brazil were well publicised. However, the exact mechanism by which the virus crosses the placenta and infects foetal brain tissue is still being studied. One theory involves the antibody-dependent-enhancement phenomena best known to occur in DENV: where non-neutralising antibodies from previous infections enhance viral replication. Both Zika and Dengue viruses share the Aedes aegypti mosquito vector, and are known to co-circulate in Brazil so it has been posited that pre-existing DENV antibodies may be enhancing viral replication in congenital Zika cases.
The findings
From a Thailand cohort containing 16 Dengue-infected children, researchers gathered plasma and peripheral blood cell samples from acutely infected and convalescent patients. Preliminary testing revealed that plasma from patients convalescing from a secondary Dengue infection bound both DENV and ZIKV, but when neutralising ability against Zika strains HD78788 and PF13 (Africa and French Polynesia respectively) were assessed, the investigators found ZIKV neutralisation to be far less efficient than that of DENV.
If the DENV immunoglobulins are cross-reactive but not neutralising, are they promoting antibody-dependent-enhancement? Moving to a human myeloid cell line U937, which is unreceptive to infection in the absence of ADE. Zika and DENV strains were incubated in DENV-infected serum before addition to the cell line. The resulting infection rate increase in the treatment arm exceeded 100 fold for both Zika viruses, indicating cross-reacting antibodies for DENV were enhancing Zika infection.
The investigators moved onto a panel of human monoclonal antibodies specific for various areas of the DENV envelope protein: one group bound the fusion-loop epitope (FLE), another were specific for the envelope but not FLE, and the remaining 'EDE' third bound intact viral particles with the ability to neutralise infection. Comparing the binding of these anti-Dengue envelope immunoglobulins between the two Zika viruses found the majority of FLE and non-FLE monoclonal antibodies cross-reacted with ZIKV, whilst binding to intact viral particles was variable. When Zika virus was cultured with the first two groups of monoclonal antibodies, infection rates of human myeloid cell lines rose significantly. In a final experiment the team of researchers investigated the EDE group which did not enhance infection, incubating Zika virus with both anti-Dengue patient sera and the various monoclonal antibodies. This study demonstrated that EDE monoclonal antibodies inhibited Zika antibody-dependent-enhancement of infection and protected cell cultures in vivo.
Criticism and Caveats
I found myself admiring the simple but elegant experimental design show by this research: each experiment was run with a positive and negative control alongside a series of dilutions. None of the techniques used are new: they're all time-tested virological assays which show that good science can be achieved without the use of the latest gene editing system. I also enjoyed seeing research that used neutralisation and infectivity assays to measure viral load, as opposed to the more common PCR to amplify infected cell RNA. The underlying assumption behind this being increased viral RNA results in increased viral egress, which does logically follow but has not been definitively confirmed for Zika.
A key assumption of this research is that cohort members were not previously exposured to ZIKV, and the results heavily rely on the fact no reports were made during sampling. Zika virus exposure cannot of course be formally excluded. I also feel the research would have benefited from using ZIKV strain from Latin American, as opposed to the African and French Polynesian strains featured but that's slightly pedantic. However, the article lays a solid basis for the ZIKV ADE hypothesis in cell cultures, which will now need to be confirmed in animal models. If readers know of any literature correlating previous DENV exposure with congenital Zika syndrome I'd be very interested to read it. Unfortunately accurate serological testing of this nature is made difficult by the very cross-reactivity shown in this paper.
Future outlook
This
research provides new evidence for the antibody-dependent-enhancement model,
but in real world terms what do these results mean? The unfortunate answer is
several things, and frankly none of them are very good. These results have the most meaning for Brazil and Latin America where Dengue and Zika are known to co-circulate and share the same vector. There is currently a single Dengue virus vaccine present on the market – Dengvaxia, developed by Sanofi Pasteur - which proved controversial when clinical trials found the treatment arm to increase hospitalisation of children under 9 years old. These results were attributed to the vaccine priming Dengue-naive patients, which resulted in ADE when secondary infections arose.
This prompted some lively academic discussion, as Scott Halstead of the Dengue Vaccine Initiative called for "further explicit study" in light of ADE evidence, which was not kindly received by members of Sanofi Pasteur. Halstead's reply asking for "a true efficacy study" has not yet been answered and thus questions remain regarding the safety of Dengvaxia. If DENV and ZIKV are found to enhance each other in further animal and human studies, this would be catastrophic for the Dengue vaccine. The described Dengvaxia controversy limited licensing, with use advised for use only in populations with prior DENV seroprevalence of 70%. Attempting to combat these shortfalls, several biotechnology companies are currently developing second generation vaccines and future study of Dengue and Zika interactions will be needed to assess ADE risks between the two viruses.
This prompted some lively academic discussion, as Scott Halstead of the Dengue Vaccine Initiative called for "further explicit study" in light of ADE evidence, which was not kindly received by members of Sanofi Pasteur. Halstead's reply asking for "a true efficacy study" has not yet been answered and thus questions remain regarding the safety of Dengvaxia. If DENV and ZIKV are found to enhance each other in further animal and human studies, this would be catastrophic for the Dengue vaccine. The described Dengvaxia controversy limited licensing, with use advised for use only in populations with prior DENV seroprevalence of 70%. Attempting to combat these shortfalls, several biotechnology companies are currently developing second generation vaccines and future study of Dengue and Zika interactions will be needed to assess ADE risks between the two viruses.
· References
1. Dejnirattisai, W., Supasa, P., Wongwiwat, W., Rouvinski, A., Barba-Spaeth, G., Duangchinda, T., Sakuntabhai, A., Cao-Lormeau, V.-M., Malasit, P., Rey, F.A., et al. (2016). Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nature Immunology 17, 1102–1108..
2. Murphy, B. and Whitehead, S. (2011). Immune Response to Dengue Virus and Prospects for a Vaccine. Annual Review of Immunology, 29(1), pp.587-619.
3. Halstead, S.B., and Russell, P.K. (2016). Protective and immunological behavior of chimeric yellow fever dengue vaccine. Vaccine 34(14), pp.1643–1647.
4. Hadinegoro, S., Arredondo-García J.L., Guy, B., et al (2016) Answer to the review from Halstead and Russell “Protective and immunological behavior of chimeric yellow fever dengue vaccine”. Vaccine 34(36) pp.4272-4273
5. Halstead, S.B., and Russell, P.K. (2016). Response to Hadinegoro et al. Vaccine 34(36), pp.4273-4274


No comments:
Post a Comment